SpineFrontier Sterile Packaging
Status: Pre-Release
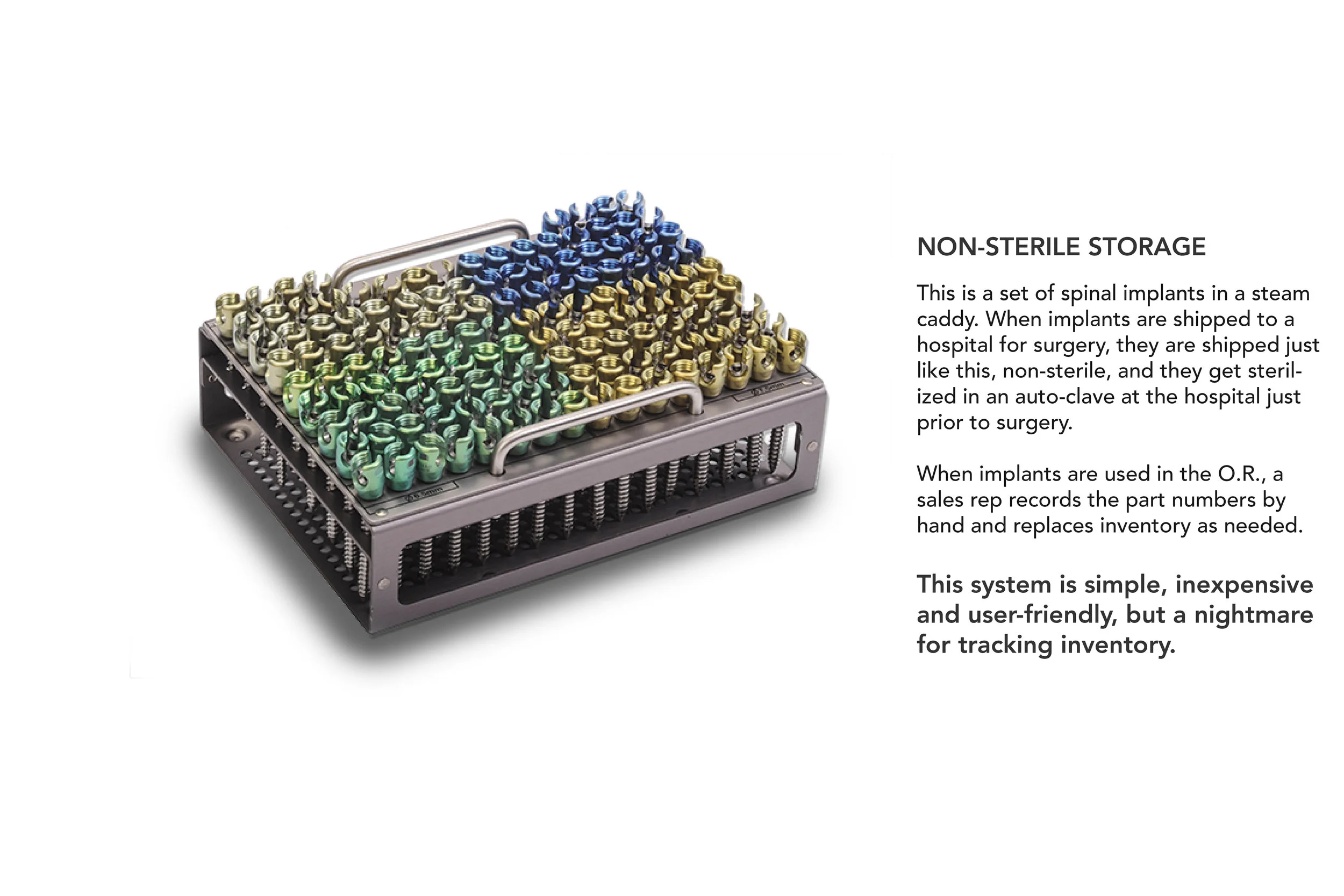
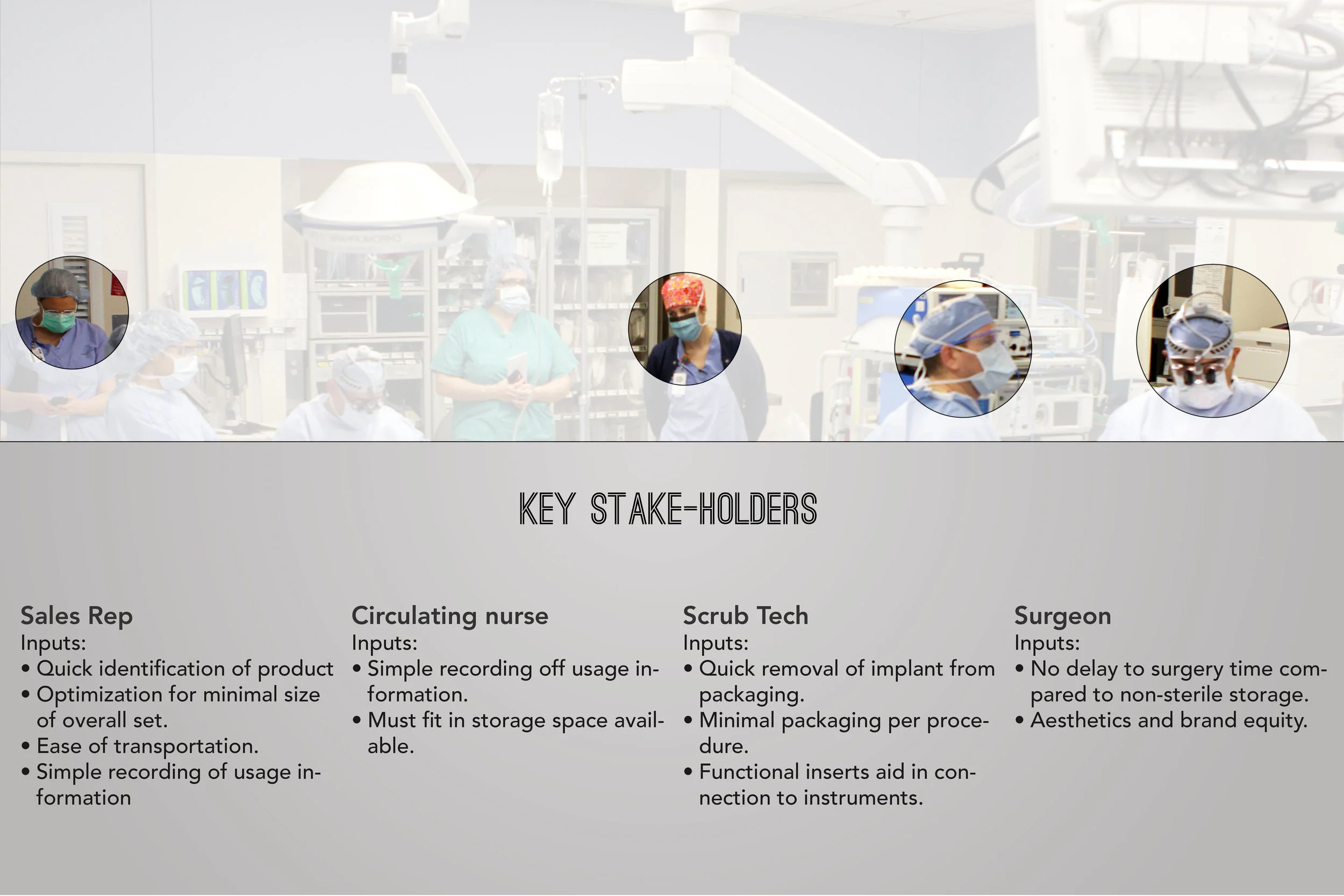
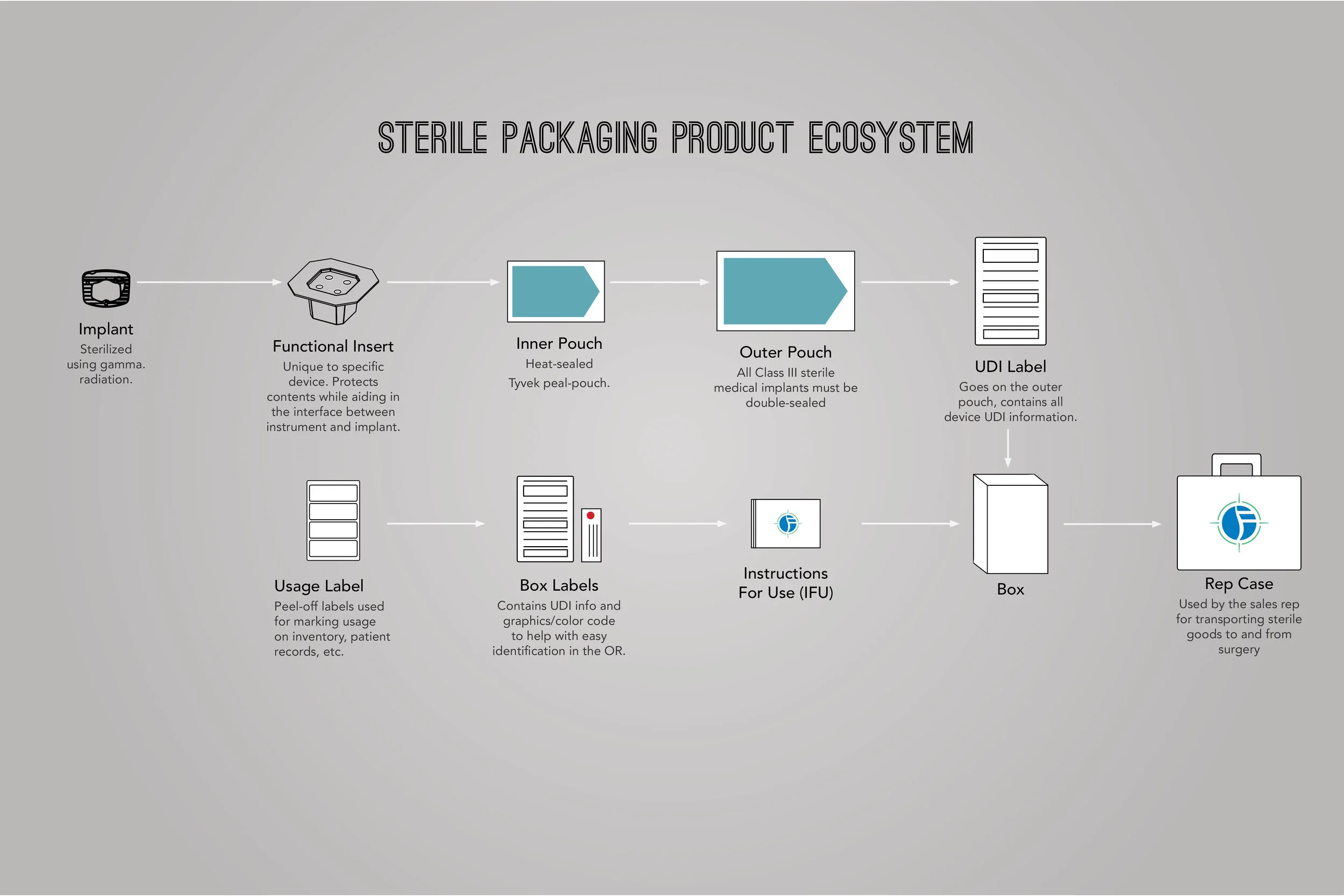
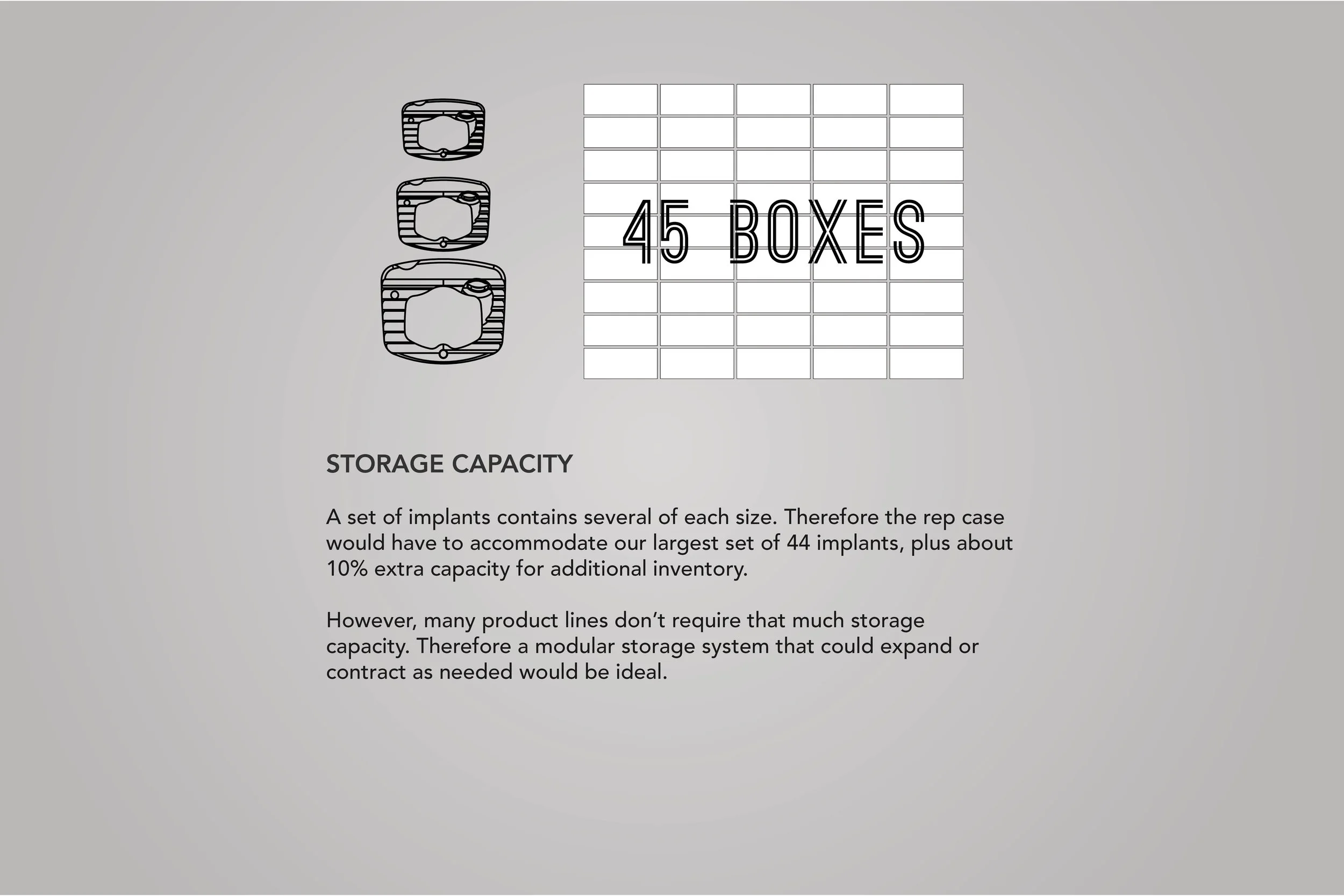
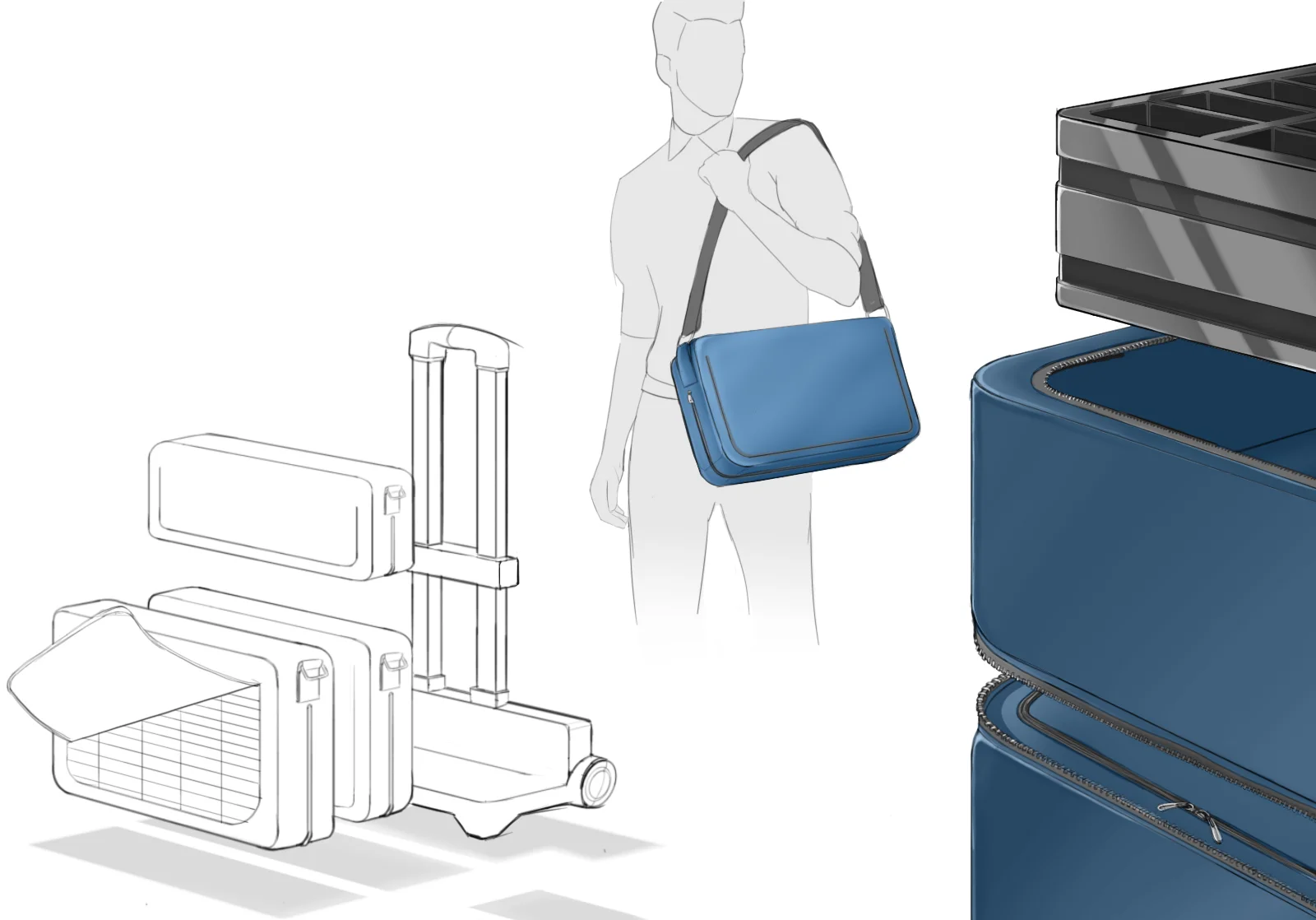
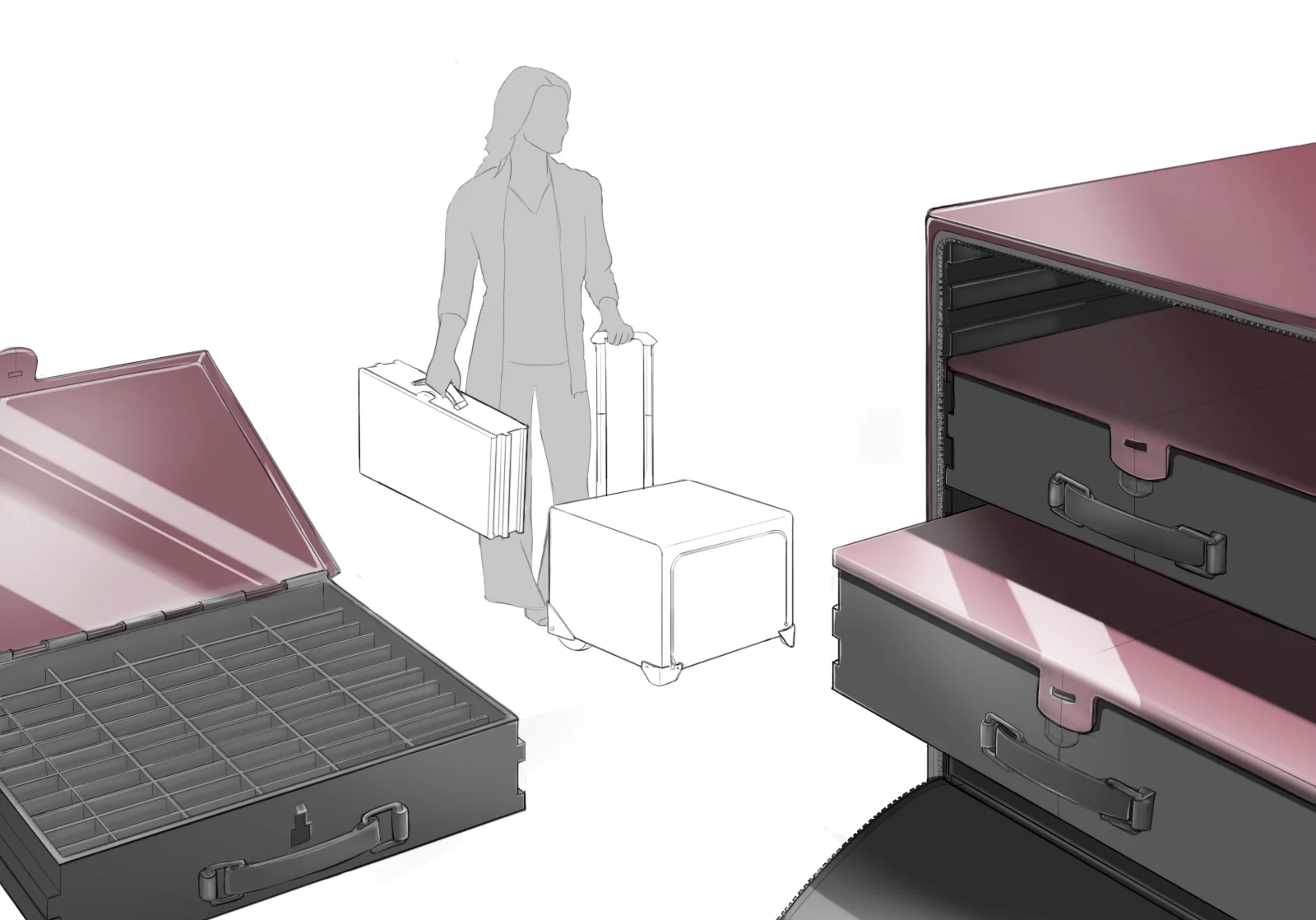
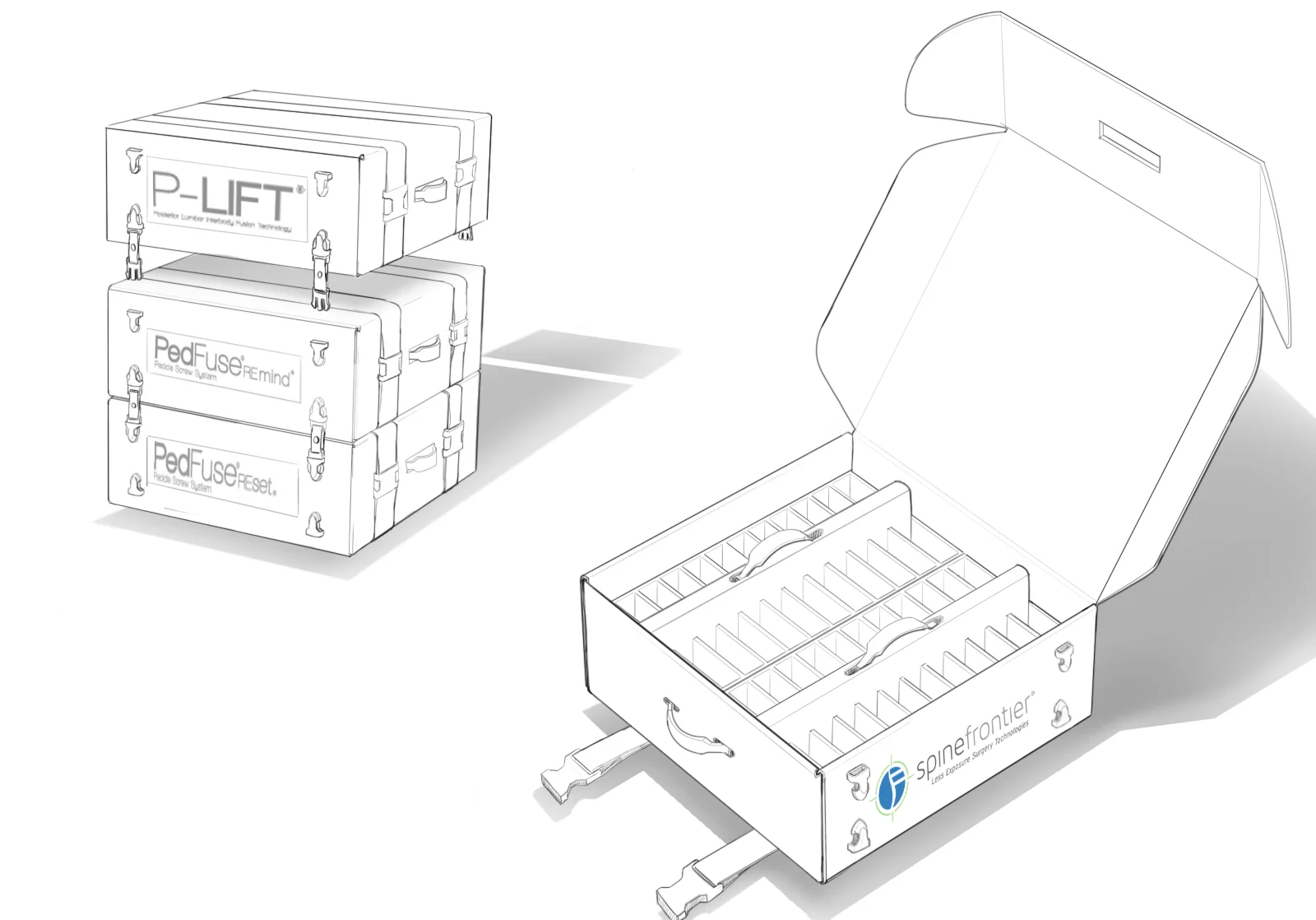
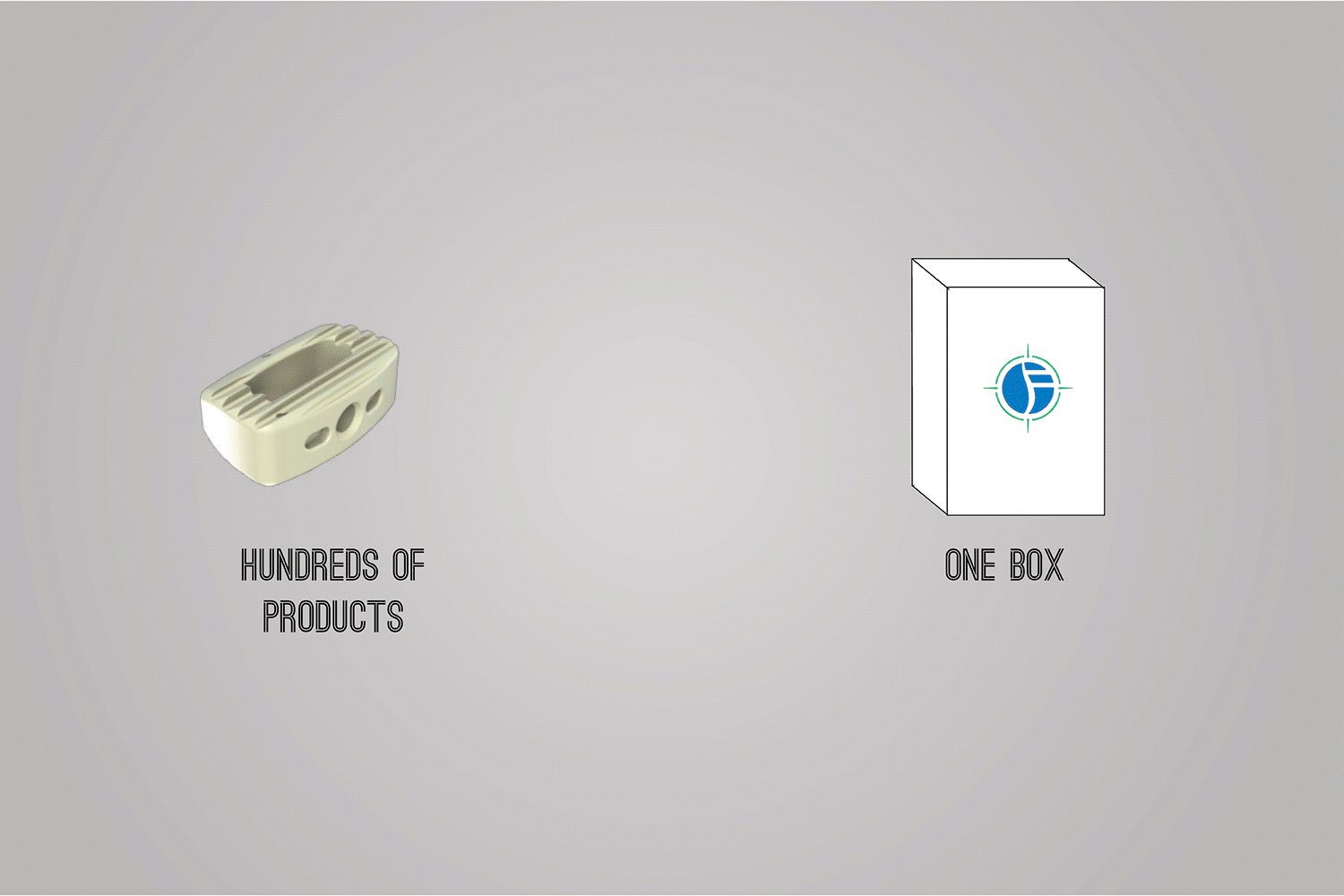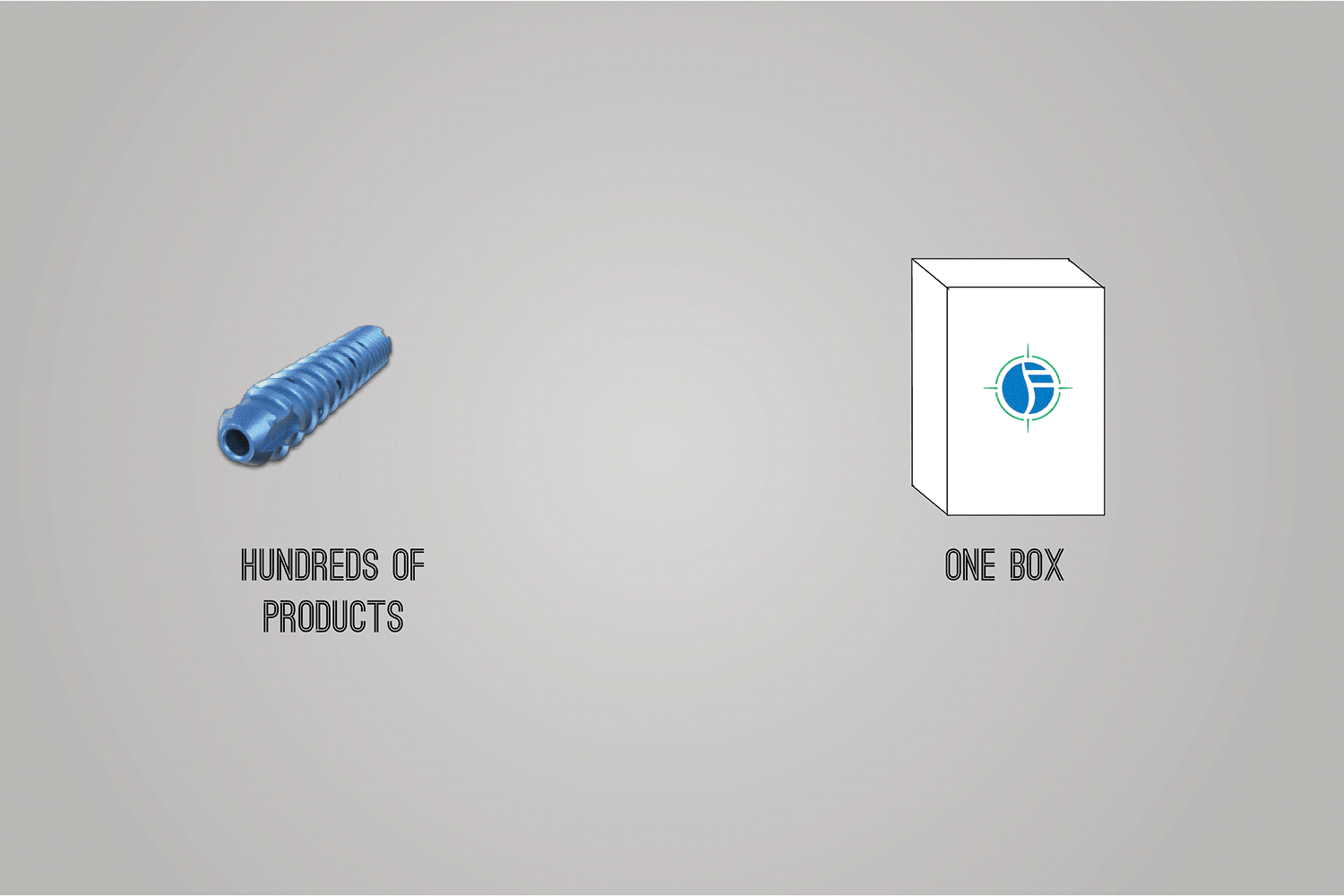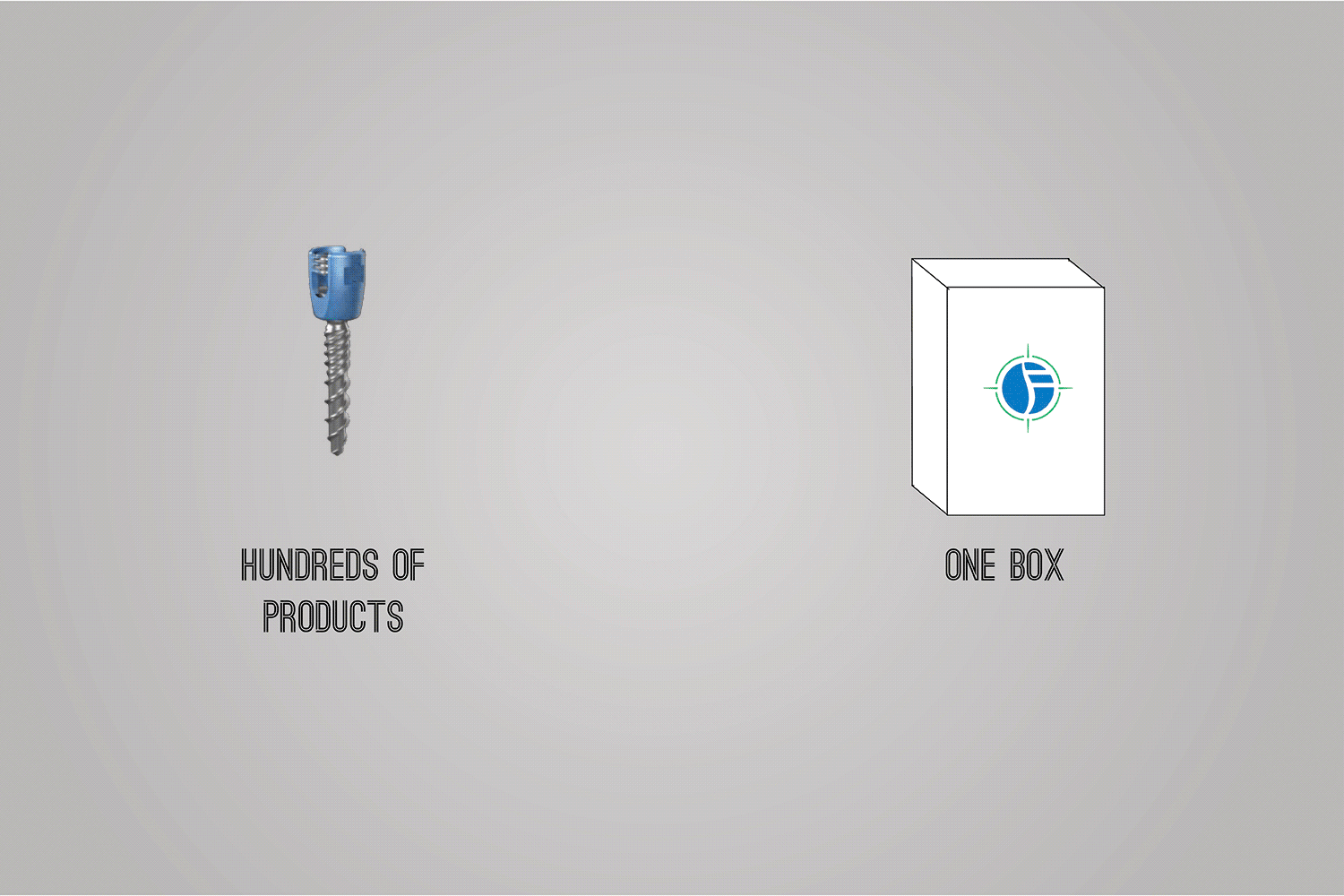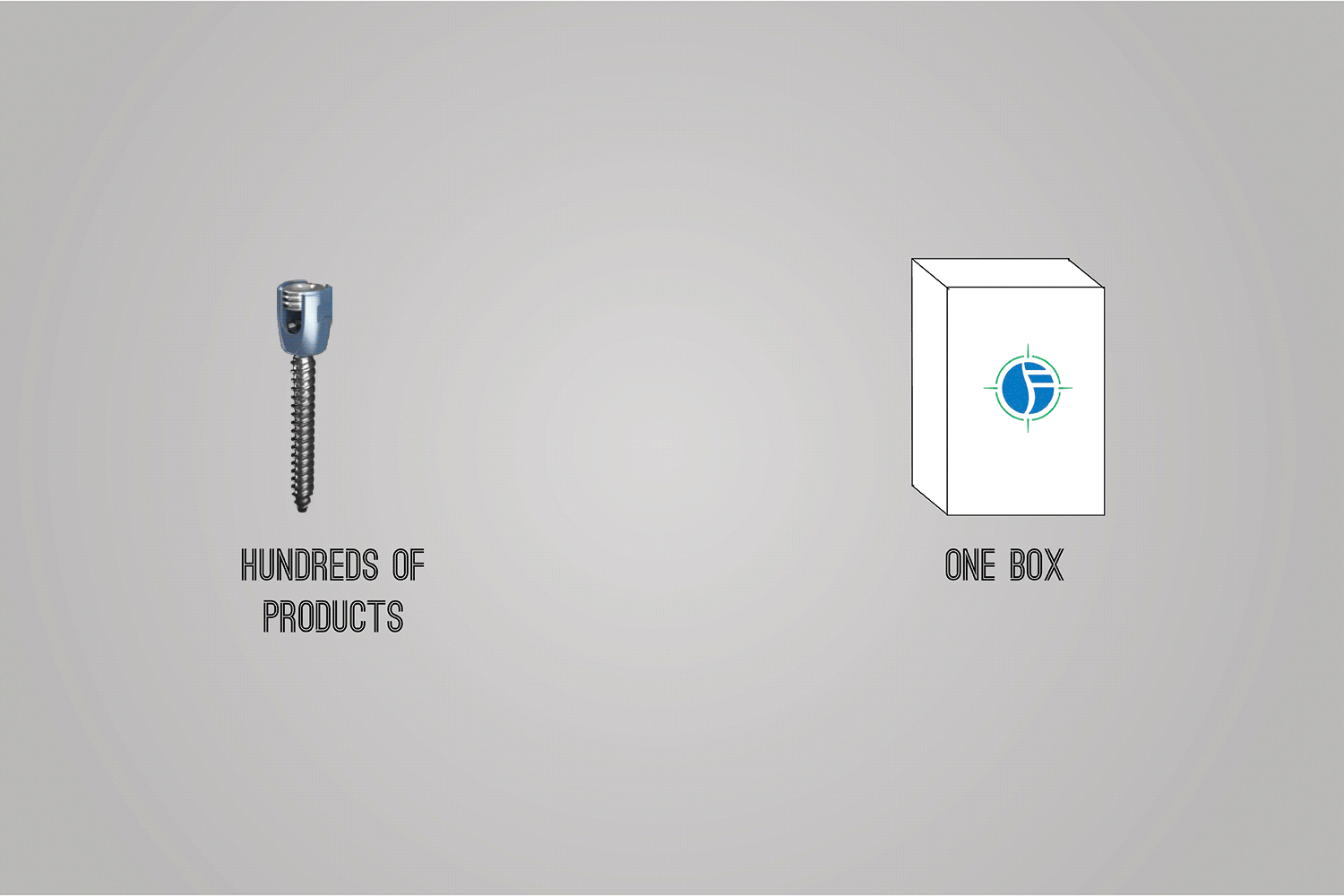
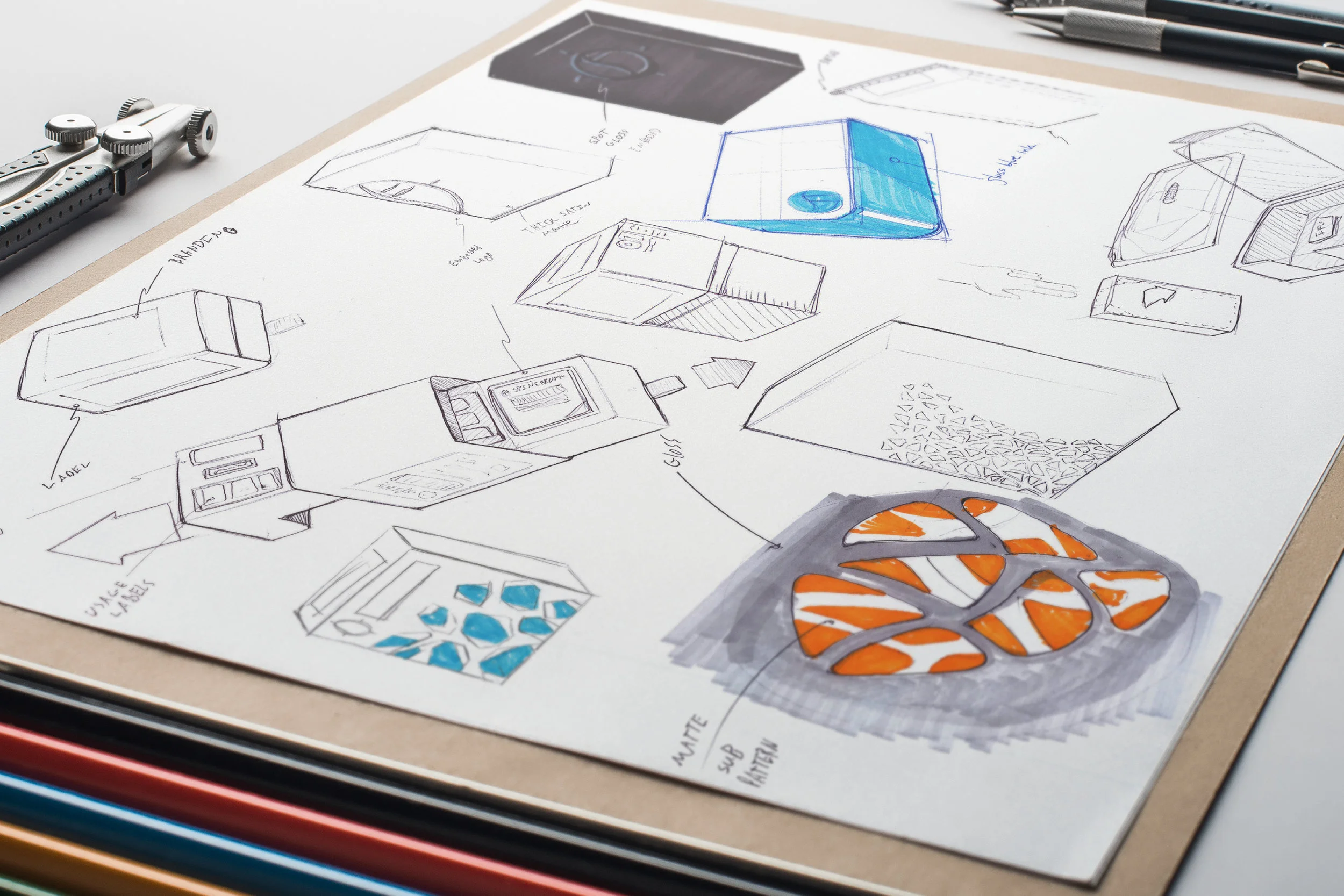
In order to comply with new FDA regulations, SpineFrontier Inc decided to dramatically pivot from a non-sterile storage and transportation strategy, to a system where every product in their catalog would be individually packaged. Adapting to a sterile packaging system would present significant organizational and design challenges, and would require a coordinated effort from the Innovation, Engineering, Quality, Supply Chain, Sales and Branding teams. I was given the opportunity to manage this project and coordinate these disparate teams, as well as design the components of the system, from the structural packaging, to the graphics, to the cases our sales reps would use to manage inventory in the field.